Choosing a Good Supplement Company
In the last installment, we explored the pros, cons, and challenges of using supplements in place of or in addition to medication. In this installment, I will focus on choosing a good supplement company, if you choose to use supplements as part of your healing journey. This will not be a list of companies I recommend, but rather guidance on how to know if a company makes safe products. It will also be focused primarily on doing so in the U.S.
In the U.S., the dietary supplement industry is regulated by the Food and Drug Administration (FDA). Because of the way supplements are viewed, regulated, and treated in the U.S., they are nowhere near as regulated as medications.
Even given this fact, though, I would argue that, overall, the dietary supplement industry is a relatively safe one. Many of those that say otherwise would prefer to see supplements treated similarly to medications. While there are potential benefits to this concept, I would argue that doing so in the U.S. would increase the cost of dietary supplements to the point they would no longer be affordable or accessible for many people.
Regulations to be Aware of
Being less regulated essentially means that the FDA is not overly concerned with the effectiveness or quality of dietary supplements. What they are concerned with is safety. This is why the first step in ensuring a dietary supplement company is a good one is to verify they are complying with FDA regulations. Generally speaking, there are two components to this: Good Manufacturing Practices (GMP) and Marketing.
GMPs are essentially legal requirements about cleanliness and safety that a company must follow if they are to be allowed to manufacture or sell dietary supplements. These include things like clean buildings and equipment, testing ingredient identity and strength, using trained personnel, having written procedures that must be followed to the letter, and having distinctly separate quality control personnel. If you would like to see exactly what these laws cover, you can see them here.
Labeling regulations apply not only to labels, but also to any marketing (online, print, social media, etc.) that is done by the company. These regulations outline exact requirements of labels even down to the order that vitamins and minerals should be listed. These laws also state that a dietary supplement company cannot make any disease claims. Essentially, a company is not allowed to hint, suggest, or say that their product cures, treats, mitigates, or prevents any disease, disease state, or symptom of a disease. These laws are found in a few different areas, but this site summarizes them well for laymen.
Verifying GMP Practices
Since GMPs are directly related to safety, this should be the first step in vetting a dietary supplement company. Luckily, there are a few easy, accessible ways to do this.
The first is through the FDA. They provide public access to a supplier compliance dashboard. The actual purpose of this dashboard is for companies to verify suppliers, but it can be used to locate information for many manufacturers as well. All you have to do is enter the name and hit search. This database will NOT include every dietary supplement manufacturer.
Keep in mind, also, that some companies may be listed under another name than their brand. And also, some companies only put their label on products and hire other companies to actually do the manufacturing. So, you may need to do a little research to find out what company is actually manufacturing the product.
This database will let you see information on inspections, compliance actions, recalls, and import violations. SO, let’s take a look at these.
FDA Inspections
When it comes to inspections, the amount or frequency does not necessarily tell you anything. But, the findings do. Ideally, a company wants to see an NAI (no action indicated, which means the FDA looked around and said everything was hunky dory). The next best is a VAI (voluntary action indicated). This means there was something(s) small that needed to be fixed, but it didn’t seriously impact safety. The worst result is OAI (official action indicated). This means that something was seriously wrong enough that the FDA had to make a stink about it. This could be a repeat minor violation or something big. If a VAI or OAI are found, there will be a brief explanation as to what was wrong.
Compliance Actions
Compliance actions refer to warning letters, injunctions, and seizures. Warning letters are not ideal but are less concerning. They are the first step in correcting possible violations. Legally, the company must take action to address and resolve warning letters. Injunctions and seizures are big worry points. This means the company did something so violating, the FDA had to take serious legal action against the company.
While injunctions and seizures are usually an immediate red flag, it is important to read the warning letters to see if they raise any red flags. Some violations such as unsanitary conditions, no written procedures, intentional use of drugs in supplements, or failure to test are definitely concerning. Other violations, though, deal more with regulatory politics. Warning letters for NAC are an excellent example of this. Many of these companies may be perfectly safe and compliant but are simply caught up in a political issue.
Recalls
Recalls are concerning, but not necessarily a giant red flag. The reality is everyone makes mistakes. You may have a perfectly clean facility, trained personnel, written procedures, and all the right testing and still, something can slip through the cracks. Even the FDA does not see a single recall as a safety issue.
What is concerning, however, is multiple recalls for the same issue. If a company experiences a recall in 2018 for salmonella, then another one in 2019, and then two more in 2020 this may be a good indicator that they are not making the corrections needed to guard against this threat, even though they know about it.
Import Issues
There are two types of import issues: import refusals and import alerts. While neither is ideal, import refusals are less concerning. An import refusal means the company thought they were buying a good product, but before it even made it to the facility it turned out to have issues. Like recalls, repeated import refusals may be a warning sign the company is not taking corrective actions needed to vet their suppliers better. Depending on the ingredient, though, it may also mean they have few to no other options for purchasing the ingredient.
Import alerts are a much bigger red flag. They usually indicate the company has so many issues with importing in the past, that anything they try to import should be looked at extra hard because there is a good chance it will be in violation.
GMP Certification
Another excellent way to verify a company is following GMPs is to find out if they have a third-party GMP certificate. Often if companies have one, they will post it somewhere on their website or they may be willing to provide it on request.
A GMP certification means an external, trained company comes in (usually annually), looks at everything the company is doing in real-time and verifies they are following GMP practices. Some of these companies have requirements that are even more stringent than the FDA.
Some companies are pretty big names in the industry and provide a lot of assurance. These include companies like NSF, Eurofins, and USP. Getting a third party from these large certifying bodies, though, can be expensive. So don’t necessarily discount small companies that have chosen to get a 3rd-party certificate from a smaller or more local source. While not as stringent, it still means someone came in and examined the company to ensure they were following GMPS.
If reputable, all of these companies get paid for the audit, not the certificate. This means they do not have a vested interest in the company passing. Instead, they have a vested interest in ensuring quality, safety, and compliance so that their name means something when you see it.
Labeling Regulations
Another good way to see if a company is on the up and up is to ensure they are following labeling regulations. Superficially, it might seem that labeling compliance wouldn’t have much impact on the actual quality or safety of the supplement. However, if the company is blatantly ignoring or violating laws on their label, it always makes me worry about what other laws they might be blatantly violating or ignoring in their manufacturing.
One of the major red flags is a company that makes claims on its labels, social media, website, or other marketing. Remember, if the company is based in the U.S. or sells its products in the U.S. it cannot say its supplement treats, cures, etc. anything. As I pointed out in my previous blog, I feel this idea is erroneous, but it is still law. And usually, a safe company that is concerned about quality is also concerned about following the law.
If a product claims to cure cancer, unclog arteries, treat arthritis, or replace insulin, I would suggest steering away from it. Instead, look for phrases like supports immune system or maintains joint health. These phrases are much vaguer, but they are legal.
Here are a few other, easy-to-spot key points in label compliance. The label should have the name and address of the company that manufactures or sells it. It should say somewhere on the product that it is a supplement. It should also tell you how much of the product is in the bottle. Also, unless it is something like a protein powder or meal replacement, most dietary supplements should have a Supplements Fact Panel as opposed to a Nutrition Facts Panel.
Vetting for Quality
While a compliant company is a safe company, it does not always mean it produces effective, quality products. Judging quality is much harder and more subjective.
Testimonials and recommendations are great, but verify where they are coming from and if they were paid for. Many companies use testimonials as part of their advertisement or pay influencers to sell their product. Some professionals (such as functional medicine doctors or herbalists) may also make solid recommendations for quality products. But, again, check to see if they are getting a kickback from the products. While sources independent from the company are harder to find, they are much more trustworthy.
If the company is large or makes a trademarked, proprietary blend, they may have researched the blend. Often, these studies will be available in some way on the company website or can be found in places like google scholar or PubMed. These studies are often paid for by the company, but if the company and researcher are ethical, the study will be independent and unbiased.
Even if the company does not have research studies available for their products, there may be research or monographs available for similar products. While this won’t prove that company’s formula is necessarily effective, it can provide some good clues.
Monographs are summary glimpses of what a particular supplement is used for and may include information about dosage, safety, parts of the plant to use, and other key information. One of the most trusted sources for monographs is the Herbal Medicines PDR. (I also provided a PDF link in the last blog to an outdated edition). If you are looking for information on vitamins and minerals, the National Institute of Health provides this resource.
Essentially when looking at the research or monograph, you are verifying the item has been found to be helpful for what the company says it is using it for, it is not combined with anything contraindicated, the way it is prepared (tincture, dry extract, etc.) or parts of the plant used (root, leaves, etc.) match what should be used, and the dosage and frequency are within recommendations. Research and monographs are simply guidelines, though, so some variance is to be expected. Be concerned when you see extreme variances, though.
Another way to get a snapshot of quality is to see what types of products the company manufactures. For example, if a company’s entire product line is herbal tinctures, but they also sell a single powder multivitamin, you may want to research that multivitamin a little harder. On the surface, it appears outside the company’s expertise, meaning the company may not yet have the knowledge, experience, or machinery needed to manufacture a quality product.
While it may be biased, it is also beneficial to check out the supplement company’s website. You can find a lot of valuable, and sometimes unexpected information here. Don’t be swayed by general platitudes or a pretty website, though. Look for detailed information, certifications, and frank discussions about quality. For example, we are committed to quality is a meaningless statement. But, an overview of the testing they complete on their supplements can be insightful.
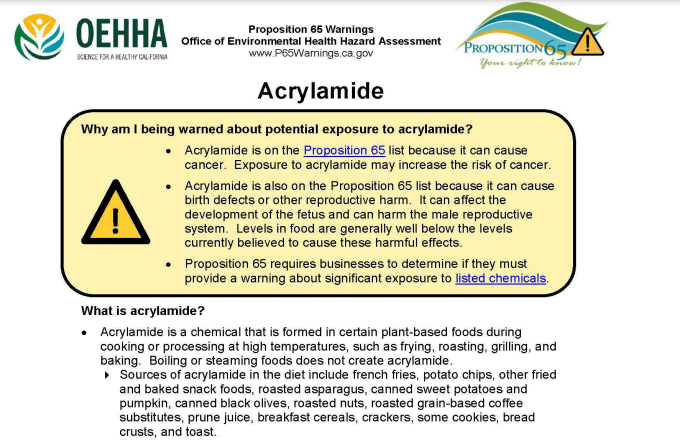
Proposition 65
While not reflective of quality or safety, I did want to mention the presence of Proposition 65 warning labels on dietary supplements. For those that do not know, Proposition 65 is a California law that requires all companies that sell anything in California to place a warning label on the product if it contains 1 of almost 900 chemicals. These warnings are frightening and essentially state that the product will cause cancer or birth defects.
While it would seem that this law would directly speak to safety, in reality, it does not. The products on the list of “chemicals” include natural plants or plant components such as pulegone (naturally found in many plants in the mint family), THC (naturally found in hemp), and even goldenseal root powder. Additionally, there are no limits for many of these chemicals and when there are limits, they are set excessively low.
Lead is the most common reason that herbal supplements contain a Proposition 65 warning. In California, the limit for lead is set 1,000 times lower than the amount known to cause harm. It is so low, in fact, that a small serving of spinach or even an orange may exceed set limits.
Because of the nature of the Proposition 65 law, a company will not be punished for placing a needless label on its product. But they can easily be fined millions of dollars for failing to have a label. What all of this means is that a proposition 65 warning label on a dietary supplement does not necessarily mean anything. If you are concerned, though, many companies provide information about the reason for the warning on their website. Some smaller supplement companies may even possibly provide you with the test results for a specific lot of product if requested.
Finding the Right Company for You
Finding the right dietary supplement company and product is about more than safety and quality, though. It is also about finding a product that works for you. Do the research, if you can. But also, listen to your body and how it is affected by the supplement you are taking. This may mean adding only one supplement at a time and then waiting a few weeks. It may also mean tracking symptoms and side effects closely at first to see how your body is reacting.
Remember, also, that not all supplements are created the same. Even if a product has the same component and the same dosage, it may affect you differently. This may be due to the manufacturing process of the supplement, the addition or absence of “filler” agents, the form of the supplement (gummy, capsule, etc.), or even how the ingredient was harvested or created. So, if you find something that works, stick with it.
It is a good idea, though, to check in now and again to make sure the supplement keeps doing its job. Sometimes our bodies become so used to a supplement that it becomes less effective. If this happens you may need to increase dosage, take a break, or find a new option. Sometimes how the supplement is manufactured changes and your body just doesn’t respond the same anymore.
There are many things to consider when trying to find a safe, effective supplement from a reputable company. Taking time to do a little research and making sure you listen to your body will help you find the answers you need.